Research and Teaching with Animals
Research and Teaching with Animals is governed by the Care and Use of Animals Policy. This policy applies to all research, teaching, and testing involving animals:
- conducted by University of Regina Faculty, Staff, and Students;
- undertaken under the auspices of or in affiliation with the University of Regina; or
- using University of Regina equipment, facilities or resources.
Only those who receive approval may bring animals onto University property, and animals may only be brought onto University property for use in research, teaching, and testing. This includes animals on display for events, animals involved in fieldwork off-campus and animals occupying University space (including outdoor spaces, offices, and leased space) but does not include service or support animals as governed by the Service and Support Animals policy.
Any University of Regina faculty, staff member, or student intending to work with animals in any capacity as part of their research or University of Regina program of study both on- and off-campus is required to receive approval before any work involving animals can be carried out.
The committee authorized to oversee the administrative aspects of research, teaching, and testing involving animals is the President’s Committee on Animal Care (PCAC).
Conducting animal research that has not been reviewed or approved by the PCAC is noncompliant.
Acquisition of animals for research, teaching, or testing that has not been approved by the PCAC is noncompliant.
Once approval from the PCAC has been granted, failure to adhere to the conditions of the approved protocol constitutes noncompliance.
Cases of suspected noncompliance will be investigated by the PCAC and the procedures outlined in the Research and Scholarly Integrity Policy.
Planning your study
Researchers are required to implement the 3Rs when preparing projects or courses with animals used in research or teaching. Replacement, Reduction and Refinement will each need to be addressed in your Animal Use Protocol for review by the animal care committee – The President’s Committee on Animal Care.
Replacement of sentient animals completely, or relative replacement with animals shown to have a lower potential for pain perception.
Reduction is the use of the minimum number of animals necessary to obtain adequate data.
Refinement to husbandry and or experimental procedures to minimize pain and destress.
For examples of how to you might implement each of the 3Rs in your study, see:
Canadian Council on Animal Care 3Rs microsite
The 3Rs Collaborative
Find Opportunities to help fund the replacement, reduction and refinement of animal use.
To reduce waste, promote the 3Rs, and make research more reproducible, NORECOPA has created the PREPARE Guidelines for researchers to follow in the planning of experiments.
Use the PREPARE Checklist (Doc), a fillable 2 page form to create a comprehensive step by step study plan from start to finish.
Watch a short video about the PREPARE Guidelines.
Before you submit your grant application:
As per the CCAC policy statement on: scientific merit and ethical review of animal-based research all research with Animals (vertebrates or invertebrate cephalopods) requires an independent, expert peer review of the scientific merit of the research project. If the project is funded through an independently peer reviewed competition, this requirement is satisfied if the work with animals and the model used is adequately described in the submission. If the project is funded through a non-peer reviewed source, or the funded project was modified to include animals, peer review is required. Contact Research.Ethics@uregina.ca in advance of Animal Use Protocol (AUP) submission. Obtaining peer review can be a lengthy process, plan your timelines accordingly.
Before an Animal Use Protocol for teaching or training can be reviewed by the animal care committee it must undergo Pedagogical Merit Review to determine if the live animal model is the best option to support the intended learning outcomes and whether the use of live animals is essential.
If you are planning to teach a course or offer training with live animals submit the Instructor Pedagogical Merit Review Form (Doc) to Research.Ethics@uregina.ca in advance of your Animal Use Protocol (AUP) submission.
Refer to the CCAC policy: Pedagogical merit of live animal-based teaching and training (PDF) and its FAQ for key considerations in the review of pedagogical merit.
Under Saskatchewan’s The Wildlife Act, 1998, a permit is required to conduct academic research or to survey wild species for purposes such as development pre-screening, baseline data collection or habitat assessments.
An Academic Research Permit Application is intended for use by researchers conducting plant, invertebrate or wildlife research on behalf of agencies or universities. The academic research application covers all other activities regulated under research permits, including bird banding or species detection surveys. A research permit is required regardless of whether an Animal Use Protocol is required for the project.
As you develop your study and fill in the Animal Use Protocol Form refer to the many CCAC guidelines available on their website. These are the criteria that PCAC will use to assess your application. The CCAC site is separated by General Guidelines and Types of Animal Guidelines.
Some of the more commonly used guidelines are linked below for your reference.
- Guide to Care and Use of Experimental Animals (PDF)
- Endpoints (PDF)
- Euthanasia (PDF) & FAQ (PDF)
- Husbandry (PDF) & FAQ (PDF)
- Laboratory Animal Facilities (PDF) & FAQ (PDF)
- Scientific Procedures Part A (PDF) & Part B (PDF)
- Amphibians (PDF) & FAQ (PDF)
- Bats (PDF)
- Fish (PDF) & FAQ (PDF) & Anesthetics (PDF)
- Mice (PDF) & FAQ (PDF)
- Wildlife (PDF) & FAQ (PDF)
Getting Approval - Animal Use Protocol Submission
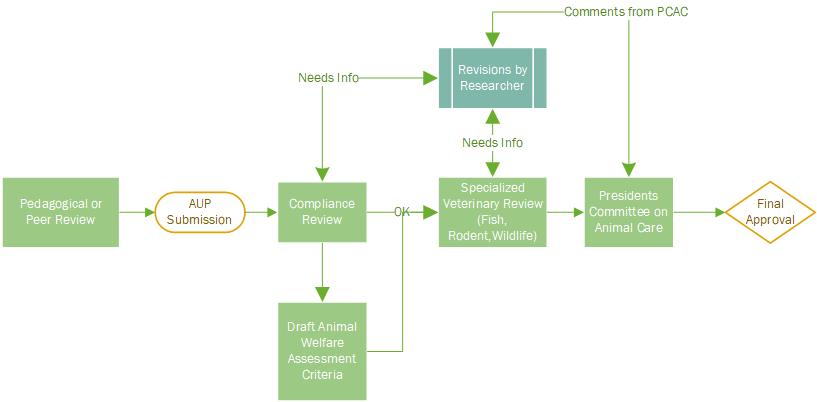
Once the project has received funding and you have obtained either peer or pedagogical merit review submit the Animal Use Protocol Form to Research.Ethics@uregina.ca & the Compliance Specialist. Applications will be reviewed by the President’s Committee on Animal Care.
Activities involving animals which are not related to a program of research, training, or a course, should be submitted using the AUP Short Form.
Any new or expired Standard Operating Procedures (SOPs) describing procedures within the AUP must be included with your submission and using the UofR SOP Template. If you require a new SOP contact your Compliance Specialist for assistance in sourcing and or drafting new SOPs.
SOP Template (Doc)
Animal Care Training
All personnel conducting research or teaching with animals must receive training in the principles of humane experimental science, and the ethical issues associated with the use of animals.
The Canadian Council on Animal Care provides core competencies and knowledge streams to meet these training needs. These modules are available through the UR Courses Animal Care Training Course which must be completed by personnel prior to their having contact with animals.
E-mail Research.Ethics@uregina.ca to be enrolled in the UR-Courses Animal Care Course. Include the AUP you are listed on, your full name, UofR ID, supervisor, any animal facilities you will be working in, or if you will be doing fieldwork and what species you will be working with.
All animal users must demonstrate that they have the practical skills necessary to competently perform their required tasks.
Each animal user including those involved in the care of animals must be listed on the Animal Use Protocol and submit the Evidence of Practical Training Form (Doc). This form should also indicate any previous training and related practical skills of the animal user.
Submit Evidence of Practical Training Forms to Research.Ethics@uregina.ca
For students and staff working in the laboratory, the Chemical & Laboratory Safety course is mandatory.
If you are supervising students or staff complete the Safety for Supervisors course.
The Health and Safety Orientation Course is a self-enrolled 30 minutes online course to provide students, faculty, and staff with general health and safety information.
The Risk Assessment/Theory Course is a self-enrolled 30 minute course to provides students, faculty, and staff with the concepts and processes of risk assessment to assist with planning work and learning activities in the safest manner possible.
After your Animal Use Protocol is approved
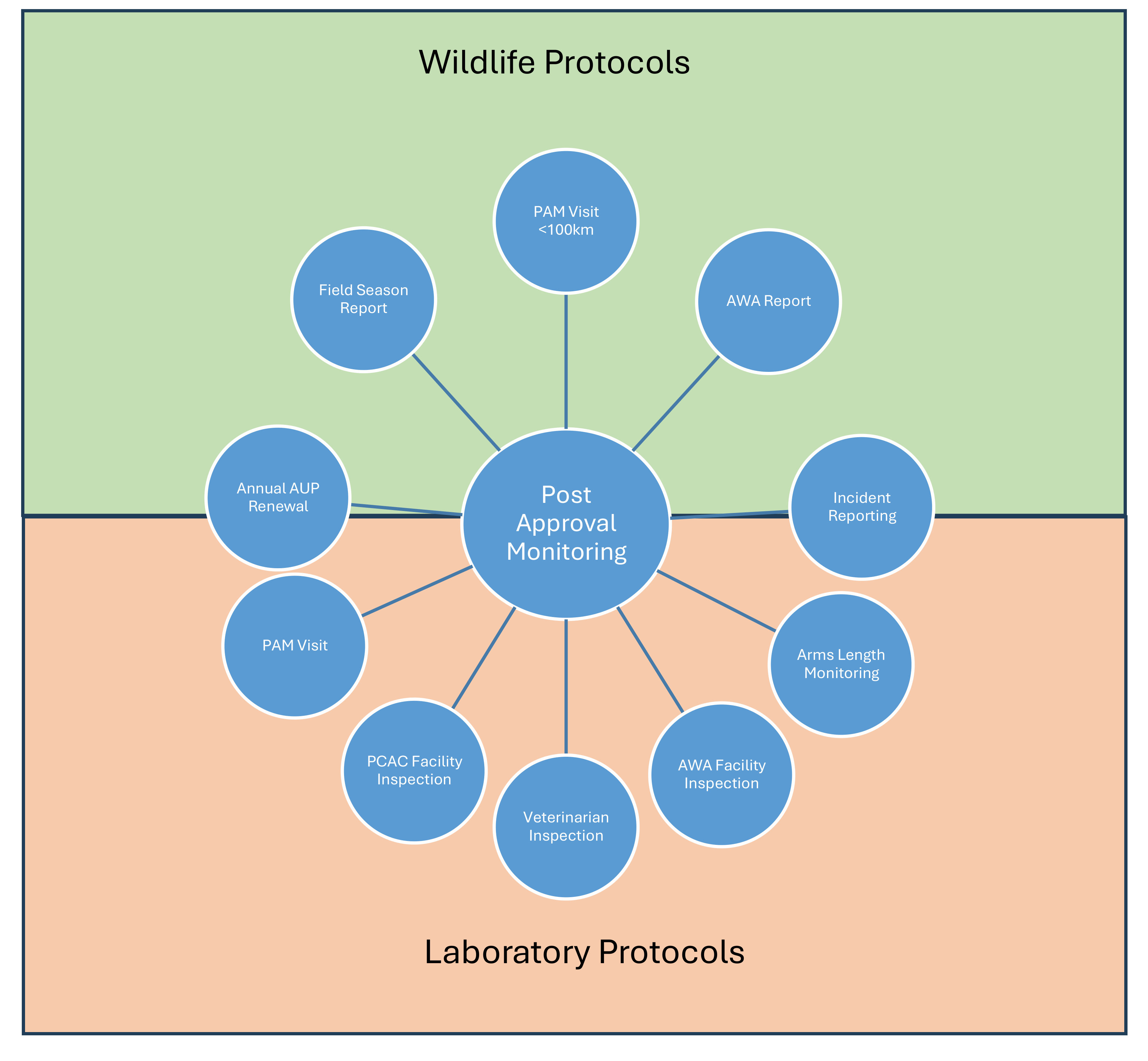
All animal purchases require advance approval from the Compliance Specialist. To purchase or acquire animals for an approved AUP, submit the Animal Procurement Form (Doc) to Research.Ethics@uregina.ca.
After approval, purchases must be coordinated via UR Stores, send the approved Animal Procurement Form (Doc) to URStores@uregina.ca
Studies are approved for one year at a time and require annual renewal if the work with animals continues into the next year. An AUP can be renewed for up to 4 years before a new AUP must be submitted. Any changes to your protocol or animal numbers requires an amendment to be submitted and approved by the President’s Committee on Animal Care in advance of implementation. Forward the completed Annual Review and Amendment Form (Doc) to Research.Ethics@uregina.ca
During the span of an AUP the project must receive a minimum of one Post Approval Monitoring assessment. If the study location is within 100km of Regina, the visit will be in person. If the study location is >100km from Regina, the researcher must submit a video demonstrating each of the procedures as they are carried out.
At the end of each field season an end of season report is to be submitted to the President’s Committee on Animal Care.
Animal Welfare Assessments (PDF) must be performed regularly for animals used for research, teaching or testing to account for physical condition, psychological well-being and the impact of experimental procedures to identify systemic welfare risks, anticipate welfare implications, and inform future decisions concerning the ethical care of animals. Part of the AUP review process will involve the review and development of animal welfare assessment criteria.
Facilities housing animals must receive an annual inspection visit by the President’s Committee on Animal Care as well as regular post approval monitoring visits and veterinary visits depending on the category of invasiveness of the procedures performed.
Each calendar year, the institution reports to the Canadian Council on Animal Care on the number of animals used, types of projects, and invasiveness of the procedures performed. Early in the new year, the Principal Investigator of each active Animal Use Protocol will receive the Animal Use Data Form template to complete. PIs will be asked to provide the following details:
- AUP number
- category of Invasiveness
- protocol description and/or Keywords
- purpose of animal use
- animal genus and species
- number of animals used in the previous calendar year
Incident Reporting
All animal welfare incidents must be reported to the President’s Committee on Animal Care to ensure that they are addressed promptly and properly. All animal incidents and how they were addressed is reported to the CCAC during assessment visits.
Animal welfare incidents are defined as events that lead to the unanticipated death of animals or that pose an immediate threat to animal health or welfare, including but not limited to:
- a catastrophic failure of critical life-support system(s)
- disregard of, or unintended failure to follow approved practices or procedures
- significant and unanticipated morbidity or mortality unrelated to the above
- serious or repeated noncompliance with CCAC standards
Report incidents using the Animal Incident Report Form (Doc) within 24 hours of the incident to the President’s Committee on Animal Care: Research.Ethics@uregina.ca